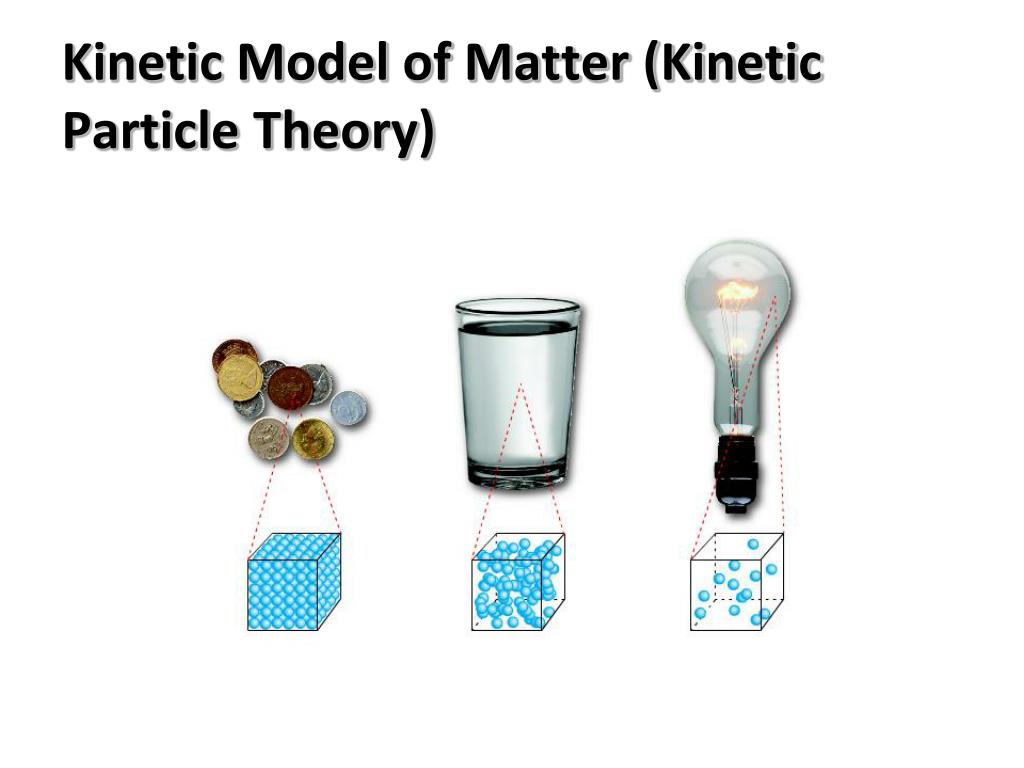
State Five Kinetic Theory Of Matter . the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. to understand the five fundamentals of kinetic molecular theory. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. Kinetic theory models the properties of matter in terms of continuous random. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. kinetic theory is the atomistic description of gases as well as liquids and solids.
from www.slideserve.com
revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. Kinetic theory models the properties of matter in terms of continuous random. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. to understand the five fundamentals of kinetic molecular theory. kinetic theory is the atomistic description of gases as well as liquids and solids.
PPT Particle Theory Model of Matter) PowerPoint
State Five Kinetic Theory Of Matter To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. kinetic theory is the atomistic description of gases as well as liquids and solids. to understand the five fundamentals of kinetic molecular theory. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. Kinetic theory models the properties of matter in terms of continuous random.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation State Five Kinetic Theory Of Matter the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. Kinetic theory models the properties of matter in terms of continuous random. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams.. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID5759037 State Five Kinetic Theory Of Matter To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. kinetic theory is the atomistic description of gases as well as liquids and solids. Kinetic theory models the. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Theory of Matter PowerPoint Presentation, free download State Five Kinetic Theory Of Matter the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. To use kinetic molecular theory to. State Five Kinetic Theory Of Matter.
From www.slideshare.net
The Theory of Matter State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. kinetic theory is the atomistic description of gases as well as liquids and solids. Kinetic theory models the properties of matter in terms of continuous random. the kinetic theory of matter. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Phases of matter PowerPoint Presentation, free download ID5071064 State Five Kinetic Theory Of Matter kinetic theory is the atomistic description of gases as well as liquids and solids. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. to understand the five fundamentals of kinetic molecular theory. revision notes on kinetic theory for the. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free State Five Kinetic Theory Of Matter to understand the five fundamentals of kinetic molecular theory. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. Kinetic theory. State Five Kinetic Theory Of Matter.
From slidetodoc.com
States of Matter Theory of Matter All State Five Kinetic Theory Of Matter to understand the five fundamentals of kinetic molecular theory. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Theory of Matter PowerPoint Presentation, free download State Five Kinetic Theory Of Matter Kinetic theory models the properties of matter in terms of continuous random. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. the particle theory of matter or. State Five Kinetic Theory Of Matter.
From dxonwlofz.blob.core.windows.net
What Is Molecular Theory Matter at Marie Craven blog State Five Kinetic Theory Of Matter To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. kinetic theory is the atomistic description of gases as well as liquids and solids. to understand the five fundamentals of kinetic molecular theory.. State Five Kinetic Theory Of Matter.
From slidetodoc.com
States of Matter Theory of Matter All State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. Kinetic theory models the properties of matter in terms of continuous random. kinetic theory is the atomistic description of gases as well as liquids and solids. revision notes on kinetic theory. State Five Kinetic Theory Of Matter.
From steemit.com
theory and changes in the state of matter — Steemit State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. to understand the five fundamentals of kinetic molecular theory. Kinetic theory models the properties of matter in terms of continuous random. revision notes on kinetic theory for the cie igcse chemistry. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT States of Matter A. The Theory PowerPoint Presentation State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. kinetic theory is the atomistic description of gases as well as liquids and solids. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at. State Five Kinetic Theory Of Matter.
From iteachly.com
Molecular Theory of Matter Chemistry Activities ⋆ State Five Kinetic Theory Of Matter Kinetic theory models the properties of matter in terms of continuous random. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. kinetic theory is the atomistic description of gases as well as liquids. State Five Kinetic Theory Of Matter.
From www.sciencelearn.org.nz
model of matter — Science Learning Hub State Five Kinetic Theory Of Matter kinetic theory is the atomistic description of gases as well as liquids and solids. To use kinetic molecular theory to describe the behavior of the macroscopic gas laws. to understand the five fundamentals of kinetic molecular theory. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules). State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Theory of Matter PowerPoint Presentation, free download State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. Kinetic theory models the properties of matter in terms of continuous random. to understand the five fundamentals of kinetic molecular theory. the kinetic theory of matter is the explanation for how. State Five Kinetic Theory Of Matter.
From www.slideshare.net
Theory Of Matter State Five Kinetic Theory Of Matter the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. the kinetic theory of matter is the explanation for how or why matter comes in many forms or states of matter, and what makes these states. Kinetic theory models the properties of. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download ID6164634 State Five Kinetic Theory Of Matter to understand the five fundamentals of kinetic molecular theory. revision notes on kinetic theory for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Kinetic theory models the properties of matter in terms of continuous random. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties. State Five Kinetic Theory Of Matter.
From www.slideserve.com
PPT Particle Theory Model of Matter) PowerPoint State Five Kinetic Theory Of Matter Kinetic theory models the properties of matter in terms of continuous random. to understand the five fundamentals of kinetic molecular theory. the particle theory of matter or the kinetic molecular theory of matter describes the microscopic properties of atoms (or molecules) and their interactions, which result in. the kinetic theory of matter is the explanation for how. State Five Kinetic Theory Of Matter.